- Expert guidance on study design
- Hundreds of models available
- Many large & small animal species available
- GLP or non-GLP studies
- Rapid study initiation - usually in under 2 weeks

Assessing a therapeutic’s potential to treat multiple sclerosis or other neuroinflammatory disorders requires models which accurately recapitulate the specific autoimmune and neurodegenerative aspects of the targeted disease. BioLegacy utilizes a comprehensive suite of Experimental Autoimmune Encephalomyelitis (EAE) models so we can select the precise platform that aligns with your compound’s mechanism of action. Our expertise across these diverse, T-cell mediated models of CNS inflammation ensures a definitive assessment of your therapeutic’s ability to modulate demyelination, inhibit immune cell infiltration, and preserve neurological function.
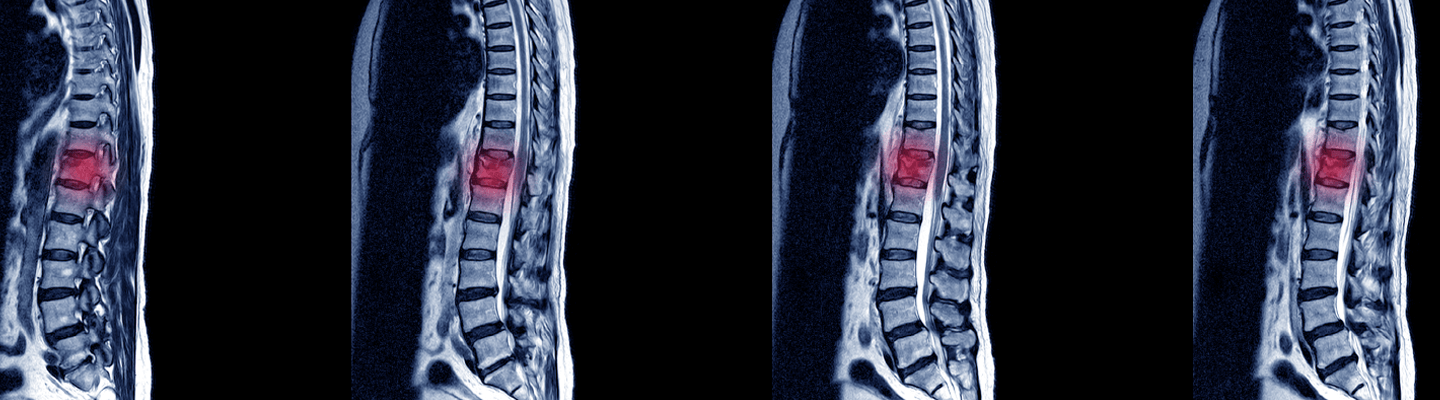
Evaluation of neuroprotective and regenerative therapeutics, devices, and surgical interventions for spinal cord injury (SCI) demands surgical precision and a deep understanding of the resulting complex pathophysiology. With a state-of-the-art veterinary surgical suite and world-class veterinary surgeons, we have the capability to implant small-scale medical devices on rodents, create highly reproducible surgically-induced SCI models, and faithfully test precise surgical interventions. Using comprehensive functional assessments and quantitative histopathology, we deliver a robust data package on your therapy’s potential to preserve tissue, limit damage, and restore locomotor function.
BioLegacy provides rigorous evaluation of anti-convulsant and anti-epileptic therapeutics through definitive, quantitative evaluation of seizure suppression. Integrating gold-standard epilepsy models with continuous, tethered video-electroencephalography (vEEG) monitoring, we provide comprehensive characterization of your compound’s efficacy in preventing seizure initiation, propagation, and generalization, delivering unambiguous electrophysiological data to advance your neuroscience program.

The efficacy of many CNS therapeutics is ultimately defined by its ability to produce a quantifiable improvement in function, and the approval of many classes of compounds requires demonstrating lack of neurotoxicity. BioLegacy provides critical behavioral and cognitive phenotyping through a comprehensive battery of validated assays. Our expertise in dissecting subtle but significant changes in motor coordination, sensory perception, and cognitive function provides a robust, objective assessment of your compound’s in vivo effect, delivering the critical functional data required to demonstrate proof-of-concept or safety and advance your neuroscience program.
A therapeutic’s efficacy against a CNS target is contingent upon its ability to achieve sufficient exposure within the correct anatomical compartment. BioLegacy’s advanced neurosurgical capabilities address this fundamental challenge, providing precise and targeted delivery of your compound. Our expertise in a range of invasive administration techniques allows for the definitive assessment of a therapeutic’s CNS activity, bypassing the blood-brain barrier to directly evaluate target engagement and downstream functional effects.
When established CNS models are insufficient to address your program’s unique scientific questions, a bespoke in vivo system is required. BioLegacy has deep experience in the design and validation of novel, custom CNS models to precisely recapitulate specific human neuropathologies. Our capabilities in advanced neurosurgery and neuropharmacology across multiple species allows us to engineer the exact translational platform needed to evaluate your unique mechanism of action and generate definitive, decision-driving efficacy data.
