- Advanced bioanalytical capabilities
- GLP or non-GLP studies
- Hundreds of models available
- Many species, from mice to NHPs
- Rapid study initiation - usually in under 2 weeks

A definitive preclinical evaluation of a heart failure therapeutic requires a model that accurately recapitulates the specific pathophysiology of either diastolic (HFpEF) or systolic (HFrEF) dysfunction. BioLegacy provides this critical distinction, offering a comprehensive suite of well-characterized surgical and medical models to induce and study both forms of heart failure. Integrating these robust in vivo platforms with gold-standard hemodynamic and functional assessments, we deliver a clear, quantitative profile of your compound’s efficacy in improving cardiac function and reversing pathological remodeling.
A therapeutic’s impact on the complex cellular and structural changes of the vasculature is a critical determinant of its clinical potential in a range of cardiovascular diseases. BioLegacy provides a definitive assessment of these effects using a comprehensive suite of robust in vivo vascular remodeling models. Our expertise in recapitulating key pathologies — from neointimal hyperplasia after injury to atherosclerotic plaque development — delivers the quantitative, mechanistic data on vascular function and morphology essential for advancing your cardiovascular program.
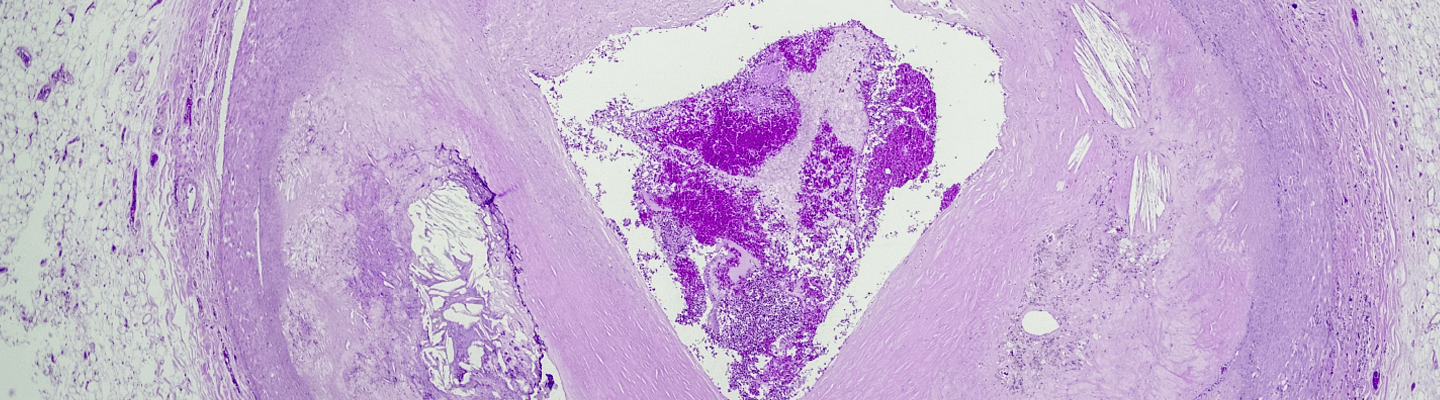
A definitive preclinical evaluation of a novel anti-thrombotic agent requires robust in vivo models that accurately recapitulate the distinct pathophysiology of venous and arterial thrombus formation. BioLegacy’s thrombosis platform is engineered to provide this critical distinction, offering well-characterized models to assess your compound’s efficacy. Providing a clear, quantitative measure of thrombus inhibition and its effect on hemostasis, we deliver pivotal proof-of-concept and safety data required to de-risk your program and advance it with confidence.
Maintaining the long-term patency of vascular grafts and access sites is a critical unmet need, driven by the complex processes of neointimal hyperplasia and stenosis. At BioLegacy, our advanced microsurgical platform is specifically designed to model these pathologies, providing a definitive assessment of your therapeutic or device’s ability to promote vascular healing and prevent graft failure. By leveraging these highly translatable in vivo systems, we deliver quantitative, mechanistic data on vessel wall morphology and blood flow that is essential for advancing your cardiovascular program.
A compound’s effect on endothelial barrier integrity is a critical parameter for determining both its therapeutic efficacy and its potential safety liabilities. BioLegacy provides assessments of in vivo vascular permeability to quantitatively address both aspects. Understand your compound’s ability to either beneficially restore barrier function in diseases like sepsis or to unintentionally induce vascular leakage as a potential off-target toxicity. Our vascular permeability studies deliver clear, functional data needed to confirm efficacy or identify potential safety risks early in development.
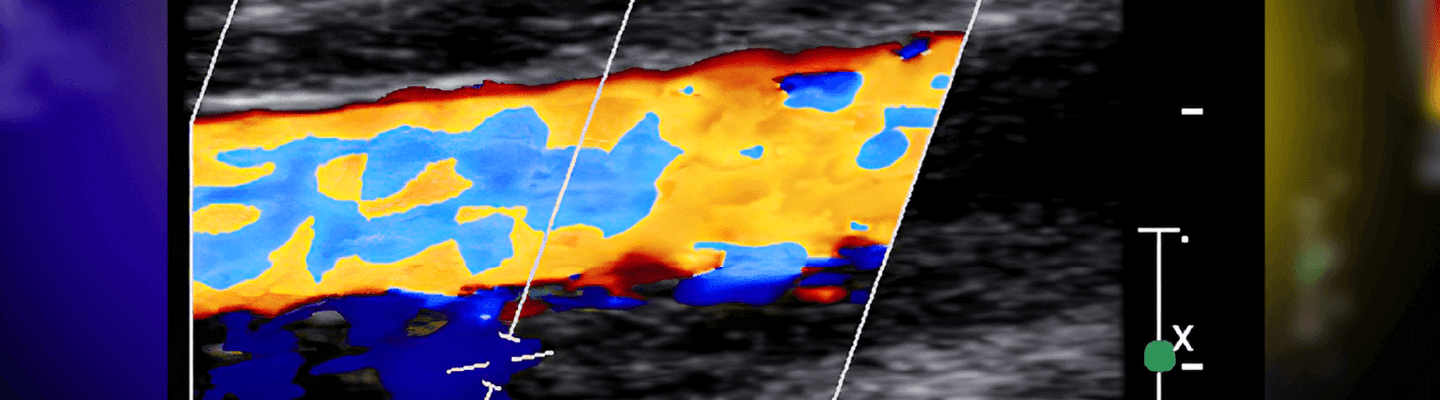
A therapeutic’s ability to restore or modulate tissue perfusion is a critical efficacy endpoint for a range of ischemic and angiogenic disease indications. BioLegacy’s quantitative assessments of regional blood flow use validated technologies, from laser Doppler imaging to colored microsphere analysis, for precise characterization of your compound or device’s impact on vascular function and tissue perfusion. We deliver the data required to demonstrate hemodynamic effect.
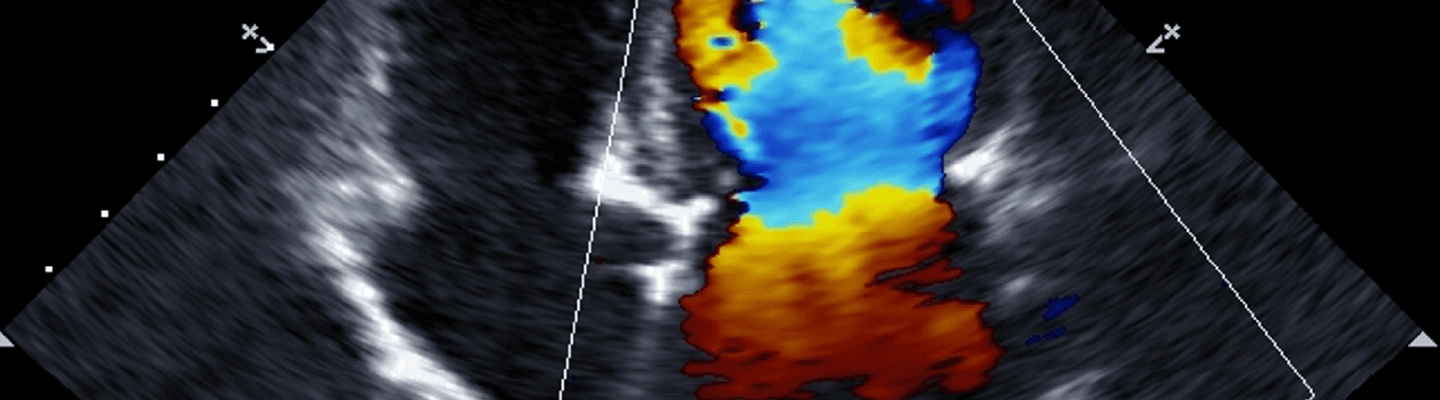
Understanding a therapeutic’s effect on cardiac performance requires a multi-modal assessment that spans from systemic hemodynamics down to intrinsic myocardial and vascular function. BioLegacy provides this definitive characterization by integrating gold-standard in vivo and ex vivo cardiovascular function assays. Our expertise in combining data from conscious, ambulatory telemetry with terminal pressure-volume loop analysis and isolated heart preparations delivers a complete, unambiguous profile of your therapeutic’s impact on cardiac contractility, vascular tone, and electrophysiology.
