- GLP or non-GLP studies
- Deep toxicological & pharmacological expertise
- World-leading microsurgical capabilities
- Advanced bioanalytical platforms
- Rapid study initiation - usually in under 2 weeks

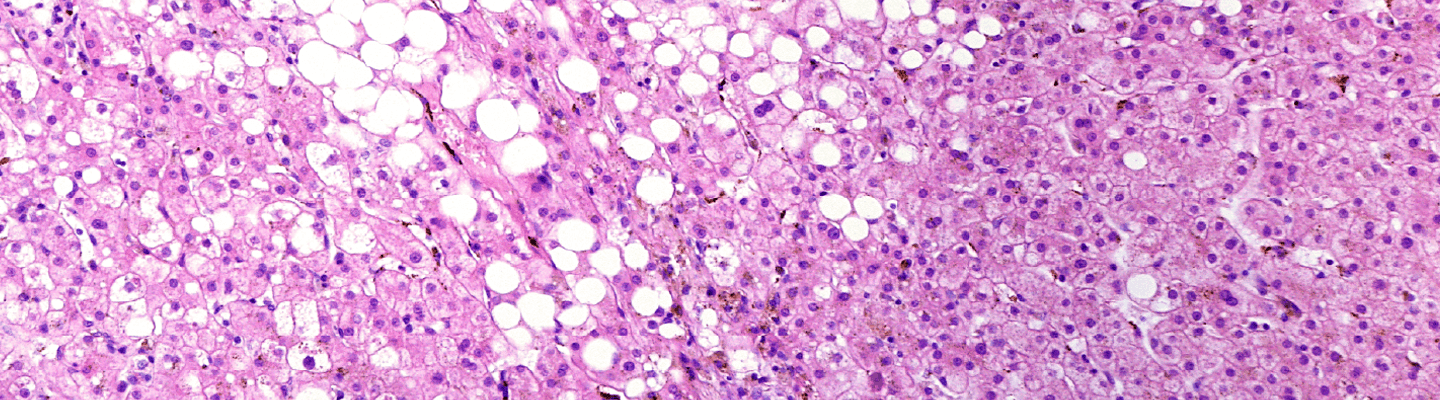
A quantitative evaluation of a therapeutic’s ability to halt or reverse the progression of hepatic fibrogenesis is a critical hurdle for any anti-fibrotic program. BioLegacy provides definitive assessments using robust, extensively validated, chemically-induced liver fibrosis models in mice and rats. Our expertise in these platforms allows for a clear characterization of your compound’s impact on hepatocyte injury, stellate cell activation, and extracellular matrix deposition, delivering the pivotal histopathological and biochemical data required to confirm efficacy.
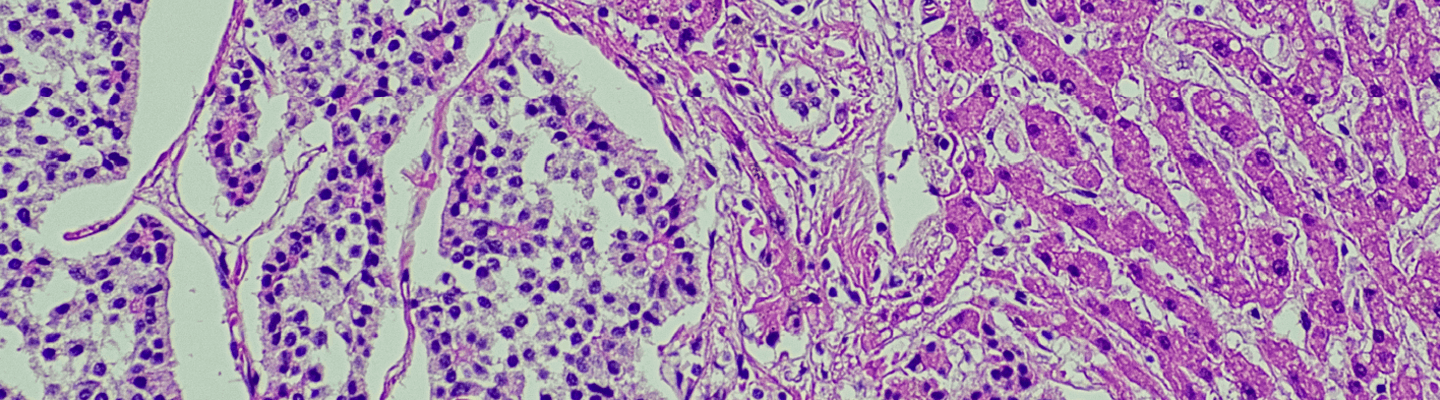
Modeling specific liver pathologies, from metastatic disease to humanized liver systems, requires the precise delivery of cells directly to the target organ. BioLegacy’s advanced microsurgical platform is engineered for this purpose, providing expert, image-guided cell inoculation directly into the liver parenchyma or portal circulation. This specialized capability allows for the creation of robust, highly translatable models of hepatic disease, enabling a definitive assessment of your therapeutic’s efficacy in a clinically relevant context.

A definitive understanding of a compound’s excretory pathways and potential for enterohepatic circulation is a critical component of any ADME package. At BioLegacy, our bile duct cannulation (BDC) platform in rats and dogs provides this essential, quantitative data with unparalleled precision. Cannulation by our expert microsurgeons allow for the direct, continuous collection of bile, enabling a definitive mass balance assessment and clear characterization of your therapeutic’s primary routes of elimination and metabolites.
