- Expert study design guidance
- “Extra Mile” service & support
- Rapid study initiation - usually in under 2 weeks
- GLP and non-GLP studies
- Expedited reporting

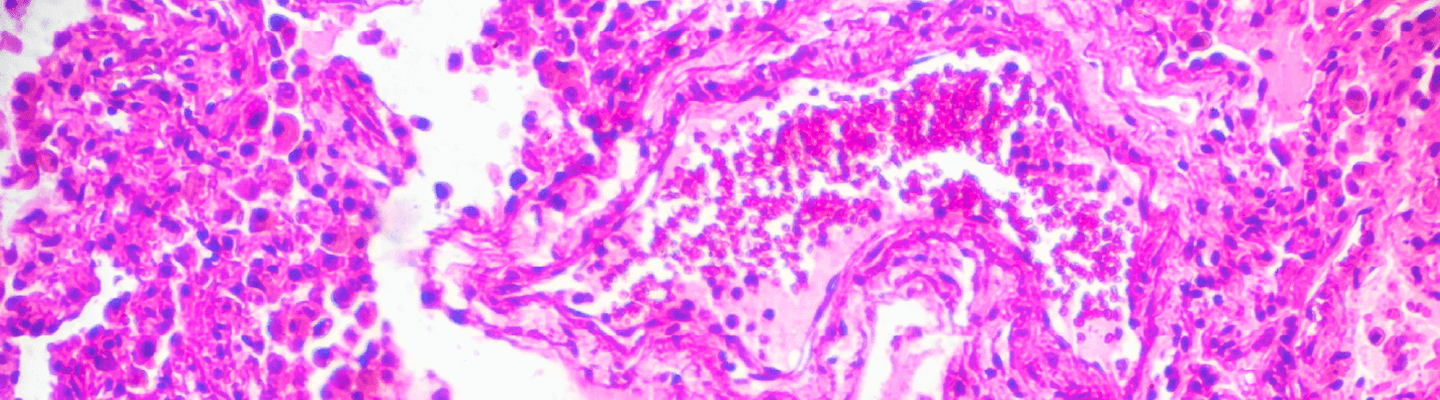
BioLegacy provides faithful modeling of the distinct pathologies of Chronic Obstructive Pulmonary Disease (COPD), from profound emphysematous changes to chronic airway inflammation, through a suite of validated COPD models to match your therapeutic’s mechanism of action. Our expertise in induced COPD models ensures a definitive, quantitative assessment of your compound’s ability to mitigate inflammation, preserve lung structure, and improve respiratory function, delivering the pivotal data your program requires.
A translationally relevant assessment of a therapeutic’s potential to treat allergic asthma requires a model that faithfully recapitulates the sensitization, challenge, and subsequent Th2-mediated inflammatory cascade seen in humans. BioLegacy provides these definitive assessments using a platform of robust, allergen-induced asthma models. Combined with our advanced bioanalytical and histological capabilities, we provide a clear, quantitative characterization of your compound’s ability to inhibit eosinophilic inflammation, reduce mucus hypersecretion, and attenuate airway hyperresponsiveness (AHR), delivering the pivotal efficacy data your program requires.

Progressive and often irreversible deposition of extracellular matrix is a key pathological driver of lung fibrosis, and one which animal models must replicate to be translational. BioLegacy provides such a model for preclinical studies: the gold-standard bleomycin-induced lung fibrosis model. This model accurately mimics the key pathological drivers of the disease such that your study delivers a clear, quantitative characterization of your compound’s ability to attenuate fibroblast proliferation, reduce collagen deposition, and preserve lung function.
