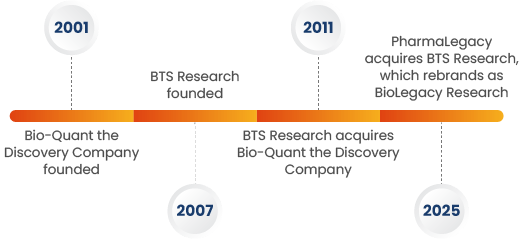
BioLegacy Research, a San Diego-based CRO with a successful 25-year history, supports in vivo and in vitro studies across a dozen therapeutic areas. We provide sponsors with access to hundreds of animal models, and have the capacity to run studies on almost any large or small animal species used in preclinical research. We have extensive experience running non-human primate (NHP) studies. With surgical precision, intelligent study design, and expert oversight, we provide our clients with data that both sponsors and regulators can rely on. With big-CRO capabilities and small-CRO agility, BioLegacy expertly manages complex drug, device, and ATMP studies.
We care about your studies!
Choose BioLegacy - Start Your Study Now

Dr. FirstName LastName
Vice President of Department
Example Pharmaceuticals