- Hundreds of models available
- Mice, mini-pigs, rabbits, NHPs, and more
- GLP or non-GLP studies
- Accelerated reporting
- Rapid study initiation - usually in under 2 weeks

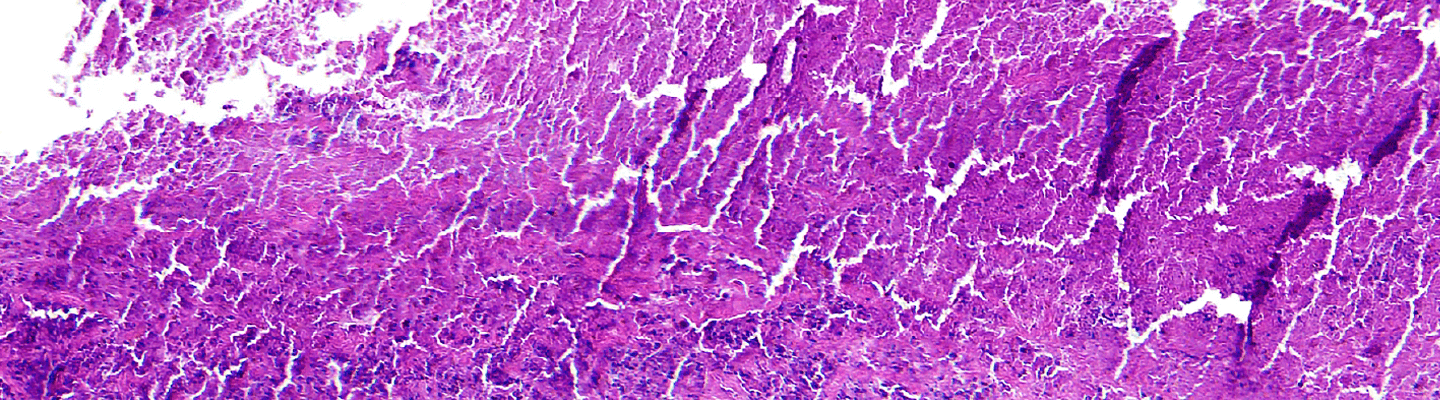
BioLegacy provides definitive, quantitative assessments of a therapeutic’s ability to accelerate the complex cascade of tissue repair. We leverage a comprehensive, multi-species platform of well-characterized dermal wound models. Our expertise in systems from diabetic ulcer models to deep wound evaluation in translational large animal species allows for a robust characterization of your compound or device’s impact on re-epithelialization, granulation tissue formation, and wound tensile strength.
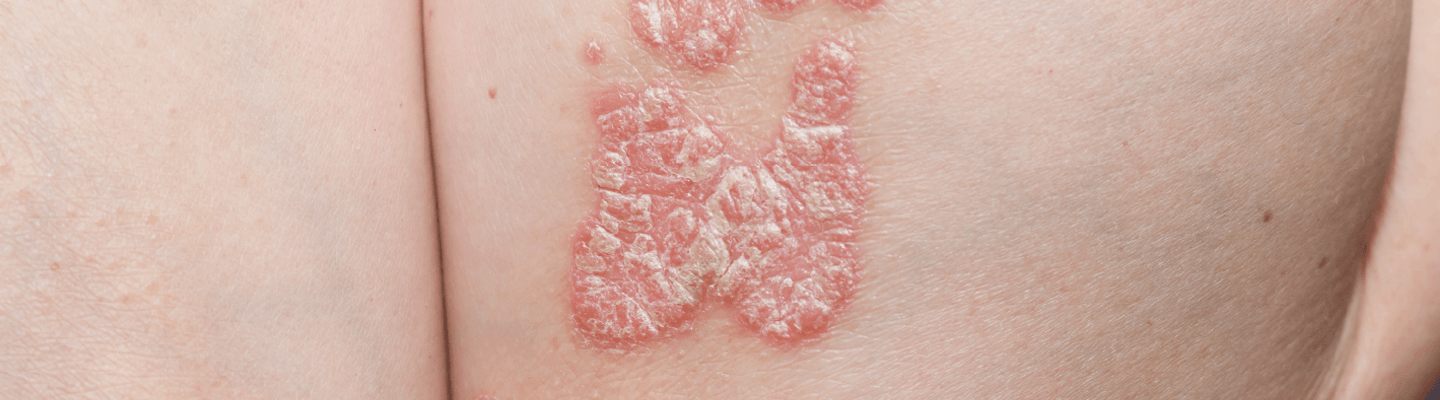
Understanding a therapeutic’s ability to impact complex skin diseases like dermatitis and psoriasis requires careful examination of distinct immune pathways. BioLegacy provides definitive assessments of immunomodulatory effects using a comprehensive suite of highly translatable in vivo models. Our expertise in platforms ranging from classic hapten-induced dermatitis to sophisticated human skin engraftment models allows for a robust characterization of your compound’s impact on epidermal hyperplasia, inflammatory infiltrates, and key cytokine axes, giving you a key understanding of its clinical potential.
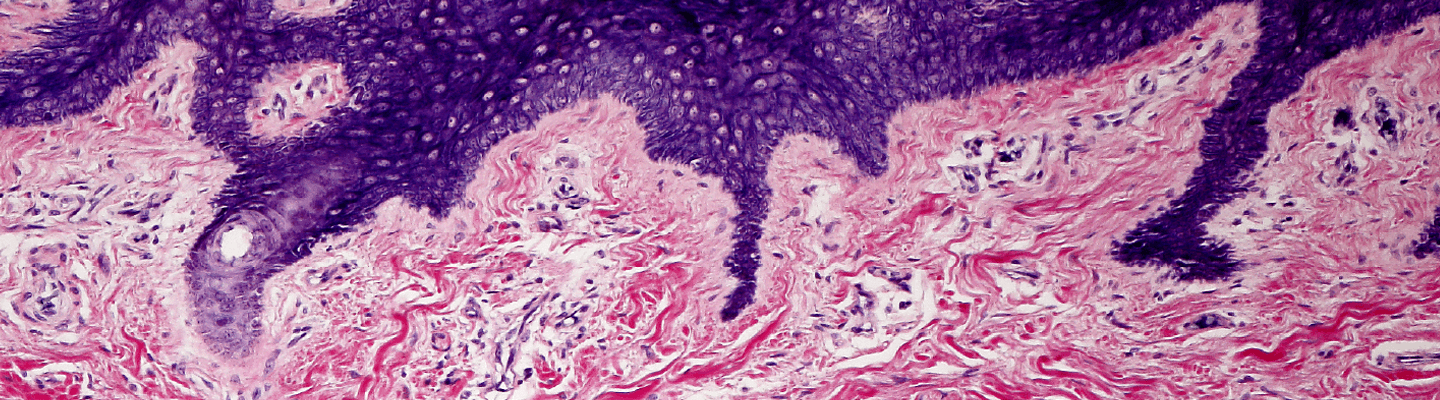
A quantitative characterization of a compound’s potential for dermal irritation is a critical safety checkpoint for any topical therapeutic or compound with dermal exposure risk. BioLegacy provides this definitive local tolerance assessment using robust, validated in vivo models that adhere to global regulatory guidelines. Our expertise in systematic scoring of erythema, edema, and other clinical signs ensures the generation of a clear, unambiguous data package to define your compound’s irritation profile and support its safe progression to the clinic.
